I recently read Billion Dollar Molecule and found myself writing paragraphs to the author in the columns so here are 6 things I learned, or relearned or found myself thinking about as I read it.
War and government are under appreciated levers in the degree of progress (and impact) they have on science
A fact people are often surprised by when they first come across it: The NIH invests ~50-60 billion into basic science every year, more than the entire ‘science’ venture ecosystem combined and then some.
World War 2 and Merck:
Bell labs is emblematic of the phenomenon of technical teams driven by the moral urgency of war and government contracts in producing frontier solutions across defense and compute.
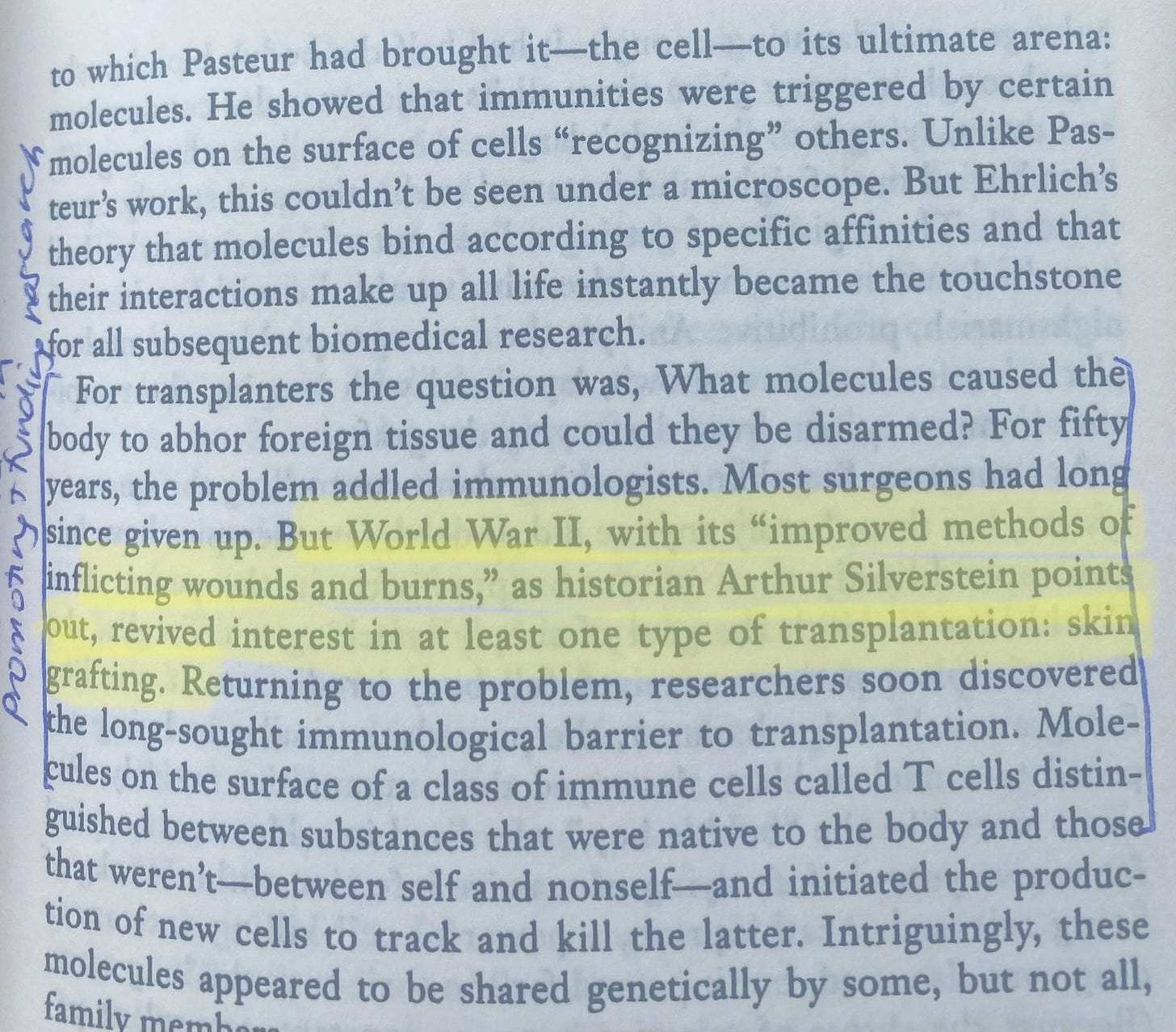
In this book, they cover the historical parallel in biotech: companies like Merck. Driven by the moral urgency of WW2 inflicting burns, there was a mounting interest and available funds to drive research in skin grafts and successful transplantation methods that the body did not reject.
The residuals of this tail wind is where Tom Starlz, Stuart Schreiber and ultimately Vertex’s founder Joshua Boger are situated in. After all, Starlz close relationship with the Reagans is part of why transplantation qualified for insurance reimbursements, drastically altering the financial incentives of providing solutions for afflicted patients.
Until the past few decades, drug discovery at most pharma companies was driven by a role that I’d effectively call: The Sherlock Homes of rare biomes.
This role/team consisted of scientists traveling to gather obscure dirt samples in biomes with differentiated environmental conditions and then screening them for activity. If there were positive hits, then the search for the active molecule would begin from there. The hero’s of this book, FK-506 and cyclosporine, are emblematic of this pattern: Both were discovered in dirt samples (FK-506 on a mountain in Japan and cyclosporine near the arctic circle in Norway). Both are poisonous yet at certain dosages and in certain cases proved beneficial in fighting off the body’s hyperactive immune response to transplants.
29 years after this book was written, we are bearing the fruits of those that dared to search for therapeutics beyond nature's search space.
We take high throughput screening, computational modeling, generative biology as terms for granted today- but to search for commercial applications that benefit patients on these foundations was unheard of just a few decades ago.
Trastuzumab to HIV Protease inhibitors alike- we have concrete examples of looking beyond nature today, proof that this approach works. I recently met someone that remarked to me, “In the 70’s you could not enter the field of computer science without a PHD. For a long time now we have been there with biology. My goal would be that years from now, we have the tools where sufficient self study means that progress and discovery in biology can be done on a computer in someone’s basement.”
When I consider the motivations that push me to contribute a verse to the field of biotech, among the most prominent is I recognize how unprecedented this time in the field is. The emerging technical challenges that arise from the intersection of biology, healthcare and compute are where I hope to dedicate the coming years of my life.
Labs require far more messy manual labor than I was aware of
Most of my interactions with scientific research has been from a computational perspective: analysis and learnings from collected data so I knew only second hand (in papers and through conversation) that this was true. But rarely are people as graphic in conversation or in Nature publications as Barry Werth describes in the book.
Vega describes some of the largely unmentioned rote tasks in lab work above but the book also includes detailed descriptions of: protein broths, thymus gland soup and calf brains. Beyond the tremendous intellectual effort by the scientists here, I leave with a greater understanding and appreciation of the physical effort that precedes the entry point of computational analysis.
Are you willing to bet the next two decades of your life on a single idea?
Boger founded Vertex Pharma in 1989. The companies first FDA approval in partnership with GSK, Agenerase, was received 10 years after Boger founded Vertex in 1999.
After 20 years with the company, he retired in 2009 and stepped down from the board in 2017. 3 years after Boger’s retirement, 23 years after he founded Vertex Pharma, the company received FDA approval for Kalydeco, a breakthrough drug that treats cystic fibrosis by targeting the CFTR gene that embodies the cystic fibrosis expertise the company is known for today.
In an era of 13 person teams building billion dollar products in software, it’s a reminder that biotech does not have historical parallels of growth at that pace. Where we can reduce timelines (grant processes, procedural approval steps, patient recruitment bottlenecks) we must and there is progress being made there but year long clinical trials to measure patient reaction are necessary and time consuming by design.
Jeremy Giffon summarized this sentiment in the tweet below, “outlier outcomes often come from people that behave in outlier, abnormal ways.”
John Thomson was the scientist on Vertex’s early team that successfully conducted the crucial isolation of “pure, isolated, biologically active FKBP”, central to proving Vertex’s merit early on and building their leverage in signing a tentative commercial deal with Glaxo.
Thomson also regularly stayed in the lab for 5-6 days at a time, dozed off for 30 minutes here and there but besides that essentially did not sleep during weeks prior to deadlines, drank heavily to cope with the immense pressure, and even got into a (luckily) non fatal car accident when driving home drunk and sleep deprived from the lab. His marriage and multiple subsequent relationships were broken and his family moved back to Australia from Cambridge.
What this biography teaches me is that the path to success is often glamorized- the heroes of this story were not intermittent fasting, eating healthy, working out, sleeping enough, prioritizing community or really optimizing for the wellbeing in most aspects of their life outside of work. An obsessive drive to solve a problem is what pushed them along above all else for a period of time. Although the intensity of this schedule appears to plateu over time, that intensity of work (even if far, far more balanced than the example of Thomson from the book) should largely energize you if you are joining an early team.
Uncomfortable reality described in this book: it is extremely difficult for early stage founders to be present with family and in particular be present with children.
In the early days of Vertex, Boger’s wife rarely saw him and his baby didn’t recognize him when he was home. In an era where one partner stayed at home this likely worked more effectively but today, 2 parent working households are the American norm.
I am certain there are examples of founders that are present parents today; yet the nature of willing a company like Vertex into life (100+ hour weeks, cut throat and rigorous timelines , lack of sleep and 24/7 mental space occupied by work) meant atleast for the individuals in this book, they seemed unable to successfully manage life as a parent and early employee.
My takeaway is more so this: 30 is unfortunately not the new 20 and 50 is not the new 30. There are biological realities to the timelines of our lives and optimistically, this means that taking bigger risks when you are young seems to compound in unexpectedly positive ways including the following- it makes it easier to make time for family later in life if that is a priority for you.










Interesting read. Specifically, this line “outlier outcomes often come from people that behave in outlier, abnormal ways" reminds me of my favorite book - Barking up the wrong tree - that explores common behaviors and myths behind successful people in a data-driven manner.
QQ: What are your motivations behind working at the intersection of bio x CS?